


Radiation Basics
More about the Justice Department cover-up of government nuclear crimes.
Frequently Asked
Questions
What is Radiation?
Why is Radiation Dangerous?
The Danger with Sandia's Mixed Waste Landfill (MWL)
To fully understand the potential danger that the Mixed Waste Landfill
poses you must understand radiation. The following information is not
as difficult to understand as you may think or others want you to think.
This information is essential for discussion of the MWL and the protection
of you and your family's health.
What is Radiation?
Radiation is a form of energy that, in general, cannot be seen, felt,
or tasted. Radiation is released from the smallest (subatomic) parts of
substances called "radionuclides" and other radioactive materials.
Radionuclides are just substances, metals for example, that because of
their make-up emit radiation. Uranium and plutonium are well-known radionuclides.
Other substances like water, air and metals can become radioactive when
exposed to radiation. At which point, they too release radiation and become
potential health hazards.
Radioactive substances emit radiation because they are unstable. That means that a uranium atom, unprovoked, changes its atomic structure. This is known as a "breakdown." The figure below shows the breakdown chart for Uranium 238.
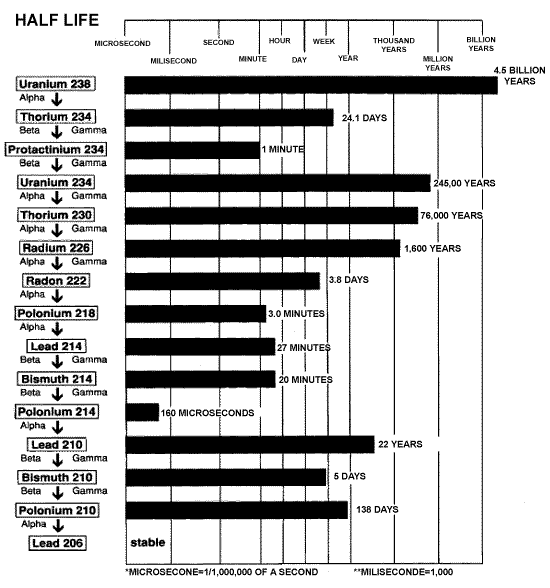
Source: CCNR website
This chart shows that Uranium 238 naturally becomes Thorium 234, which
in turn becomes Protactinium 234 and so on down the chain. Thorium, Protactinium,
and the other elements on this chart are known as the "breakdown"
or "daughter" elements of Uranium 238. The chart also shows
the half-lives of these elements. For example, Uranium 238's half-life
is 4.5 billion years. The half-life of an element is the time it takes
for 1/2 of a given mass of Uranium 238 to breakdown and become its daughter
elements. That means if you have a kilogram of Uranium 238 today, in 4.5
billion years you will have 0.5 kilograms and an array of its breakdown
elements.
You may wonder why these elements have numbers like "238" at the end of their names. These numbers are known as "mass numbers" and they represent the "atomic mass" of the element. The atomic mass is the combined number of neutrons and protons that an element has in its nucleus. For example Uranium 238 has 92 protons and 146 neutrons (92 + 146 = 238). Uranium234 has a mass number of 234. Uranium238 and Uranium234 are different "isotopes" of the element uranium. Since they are radioactive, they are known as "radioisotopes."
You will also notice that the chart above has the words "Alpha,"
"Beta," and "Gamma" listed between the elements. The
way a radioisotope transforms into a different radioisotope (e.g. Uranium238
becomes Thorium234) is by losing electrons, protons and neutrons. Some
will also emit gamma radiation. Technically radiation exists only in waveform
like gamma waves, but alpha and beta particles are typically referred
to as radiation since they are released from "radioactive" materials.
Many people outside of scientific discussions speak of radiation as including
alpha particles, beta particles and neutrons. The release of neutrons
is important in the "fission" processes in nuclear power plants
and bombs. Please understand the technical difference between particles
and radiation, but for the sake of this article gamma rays, together with
alpha and beta particles and neutrons will all be referred to as "radiation."
Every atom in the universe is made of protons, neutrons, and electrons.
The number of protons, neutrons and electrons determines what that atom
is; the numbers allow us to differentiate between oxygen, iron, and uranium.
The center of the atom, called the nucleus contains protons and neutrons,
which are orbited by electrons. Below are two representations of atoms,
one emitting an alpha particle, the other a beta particle:
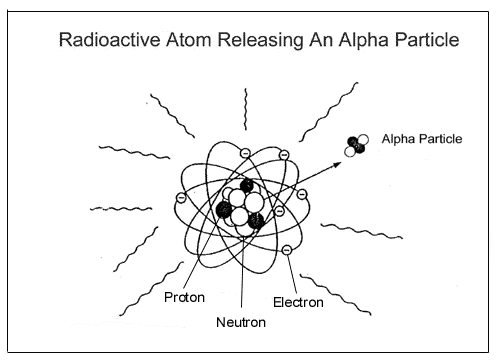
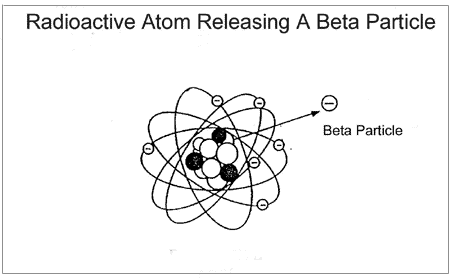
An alpha particle is made of 2 protons and 2 neutrons and has a positive
charge. A beta particle has the mass and negative charge of an electron,
but is emitted from the nucleus. Gamma radiation is another of form of
radiation. It is electromagnetic waves similar to, yet stronger than Ultraviolet
and X-rays. If you look back at the chart you will notice that a Uranium
238 atom emits radiation in the form of an alpha particle when it breaks
down to become Thorium 234. Therefore, Uranium 238 is known as an "alpha
emitter." Likewise "beta emitters" such as Thorium 234
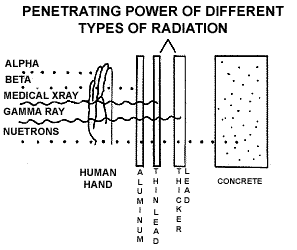
release radiation in the form of a beta particle. The figure below shows
the penetrating power of different types of radiation. Alpha particles
cannot penetrate the outer layer of your skin but can damage it. Beta
radiation can pass through the outer layer of your skin, but cannot make
it to your internal organs. Because of this alpha and beta emitters are
most dangerous to human health when they get inside your body and are
directly exposed to living cells. Radioactive elements can enter the body
by being inhaled, ingested with food or water, through a cut or wound
in the skin, or absorption. Gamma rays can pass through your entire body.
They do not travel very far through the air but they have considerable
energy and are therefore, very dangerous. (The figure below shows the
penetrating power of different kinds of radiation.)
Why is Radiation Dangerous?
Now that you know what radiation is, you need to know how and why it is
dangerous to humans and other living things. Some forms of radiation are
more dangerous than others. Some argue that there is radiation all around
us so we ought not concern ourselves with it. The concern is over the
type of radiation and the amount someone is exposed to. For example, the
spectrum of visible light is radiation, but it is not dangerous to humans.
Microwaves, ultraviolet rays, X-Rays are more dangerous. The radiation
associated with radioactive materials can be far more dangerous and should
be a concern when found in close proximity to human populations. When
we discuss the dangers of "radiation" here we are referring
to the type of radiation that is emitted from radioactive materials.
Since radiation works on such a small scale it affects the very small
parts of living things. Alpha and beta particles as well as gamma rays
and neutrons have harmful effects on cells and even the molecules that
constitute them. With its large influx of energy, radiation can alter
the state of cellular, molecular, and even atomic structures. Radiation
exposure to cells has been likened to microscopic explosions. These "explosions"
can change either cell behavior or chemical makeup. They can cause the
cell to stop operating, reproducing, and even kill the cell. If cells
in the organs in your body die faster than they are produced or if they
are unable to reproduce altogether, the result could be organ failure,
other serious health problems, and possibly death. Radiation exposure
can also cause the cell to alter its behavior. A damaged cell can produce
slightly different hormones and enzymes than it is supposed to. If this
altered cell reproduces, it could lead to the existence of millions of
such cells in one's body. This condition, known as "biological magnification,"
can cause chronic diseases and health deterioration. One of the mutations
that radiation may cause to cell behavior increases the rate of reproduction.
This "runaway" regeneration of cells in one place creates a
large mass of abnormal cells, that if unchecked will form a tumor (benign
or malignant). This same behavior is the cause of such diseases as leukemia.
Another danger of radiation exposure is the change it induces at the atomic level. Stable atoms have no charge, meaning that, as a whole, they are neutral (neither positively or negatively charged). As radiation passes through some atoms, it may cause electrons to break free. Some scientists liken this to a small-scale equivalent of a planet being forced out of its orbit around its sun because of an explosion. This action, in turn creates two ions (charged atoms or molecules,) one positive and one negative. The process of creating ions is called "ionization."
Ionization threatens human health by altering complex molecules such as carbohydrates, fats and proteins in our bodies. These substances have elaborate structures held together by shared electrons in chemical bonds. If radiation disrupts these electrons causing ionization, the complex molecules could fragment or elongate into abnormal structures. These complex molecules are essential in the proper functioning of the body. If these molecules are altered or lost it can have adverse effects on one's health. One particularly dangerous place this happens is in the proteins that make DNA and RNA. Damage to DNA and RNA has many harmful effects. It can weaken a person's defenses against disease, decrease ability to heal, and undermine one's ability to adapt to environmental changes. This process can also cause abnormal cell division (benign tumors or various kinds of cancer) and Genetic/hereditary deformities.
Radiation exposure has entirely different implications on reproductive organs and the unborn. A mutated reproductive cell has the potential of passing on the "defect" to one's offspring. This heritage will continue "forever" until the lineage dies off. An additional harmful effect of radiation on progeny may take years to surface. If recessive genes are damaged, then that "defect" will not show up until two people with the same recessive gene have a child. Genetic defects that can be passed on to your offspring include children with deformed or underdeveloped physical characteristics, children born mentally handicapped, children born with weakened immune systems, and children born with congenital diseases. Embryos and fetuses are particularly vulnerable to radiation because their cells are dividing so rapidly. Damaging a cell that early on can lead to numerous defects, syndromes and illnesses. One such disability in fetuses that can be caused by radiation is Down's Syndrome. Radiation exposure has also been known to cause sterility in men as well as making women unable to conceive or carry a child to term.
Young children in general have a high risk of health problems due to radiation exposure because of their rapid rate of growth. The chronically ill, the elderly, and the malnourished are also more susceptible to health damage from radiation because of their inability to heal as well as others. People with a family history of vulnerability to environmental or radiation hazards are also at higher risk for radiation-induced conditions.
Many radioisotopes collect in specific parts of the body. Some are able to "mimic" nutrients and substances that the human body needs and therefore become incorporated into living cells. For example, Strontium 90, with similar chemical properties as calcium is incorporated into living bone. Radium is also a "bone-seeker". Likewise, plutonium when absorbed into the bloodstream, concentrates in bones as well as the liver. The thyroid will absorb radioactive iodine. Cesium will concentrate in muscles. Any radioactive element in particulate form, that is dust, can lodge in the lungs. Uranium, plutonium, thorium, and radium are just a few of the many radioisotopes that can get lodged in your lungs. As long as these elements remain in your body, they expose your vulnerable, living tissues to the damaging effects of radiation. Some of these elements will stay where they are for your entire life and once they are in your body they may be impossible to remove.
Many radioactive substances, not only have radiological effects on human health, they are also harmful because of their chemical properties. In the same way that lead is poisonous to humans, so are uranium and other radioactive elements. For example, Uranium238 is a heavy metal and has been shown to do harm to your kidneys.
There is some debate about how much radiation is dangerous for humans. Some people believe that certain amounts of radiation are good for you, while others contend that any amount poses a potential threat. Generally the nuclear industry has pushed for loosened regulations allowing higher radiation exposure to human populations. This would allow for more radioactive wastes to be haphazardly and carelessly deposited around the country. Those that oppose the continued generation and dumping of radioactive wastes feel that the risk is too great to allow human populations and the environment to be exposed to the potential and unknown dangers of radiation. For one thing is for certain, there have been far too few health studies conducted on the health effects of low-level radiation (radiation that does not come from the inside of a nuclear reactor) for anyone to say with certainty that it is safe.
The Danger with Sandia's "Mixed Waste Landfill"
The Mixed Waste Landfill
contains an assortment of radioactive and toxic materials that have been
linked to cancer and other health problems. The landfill contains radioactive
elements such as of uranium, radium, radon, americium, cobalt, cesium,
plutonium, and polonium. These elements are dangerous due to both their
radioactive and toxic natures. For more information on the health effects
of these elements see the related section on this website Resnikoff
Report.